Science: Radiation
- As learnt in the Chemistry topic, every atom of the same element has the same number of protons (atomic number)
- However, the number of Neutrons can be different, but it will still be the same element.
- Atoms of the same element, but with different numbers of neutrons are known as ISOTOPES
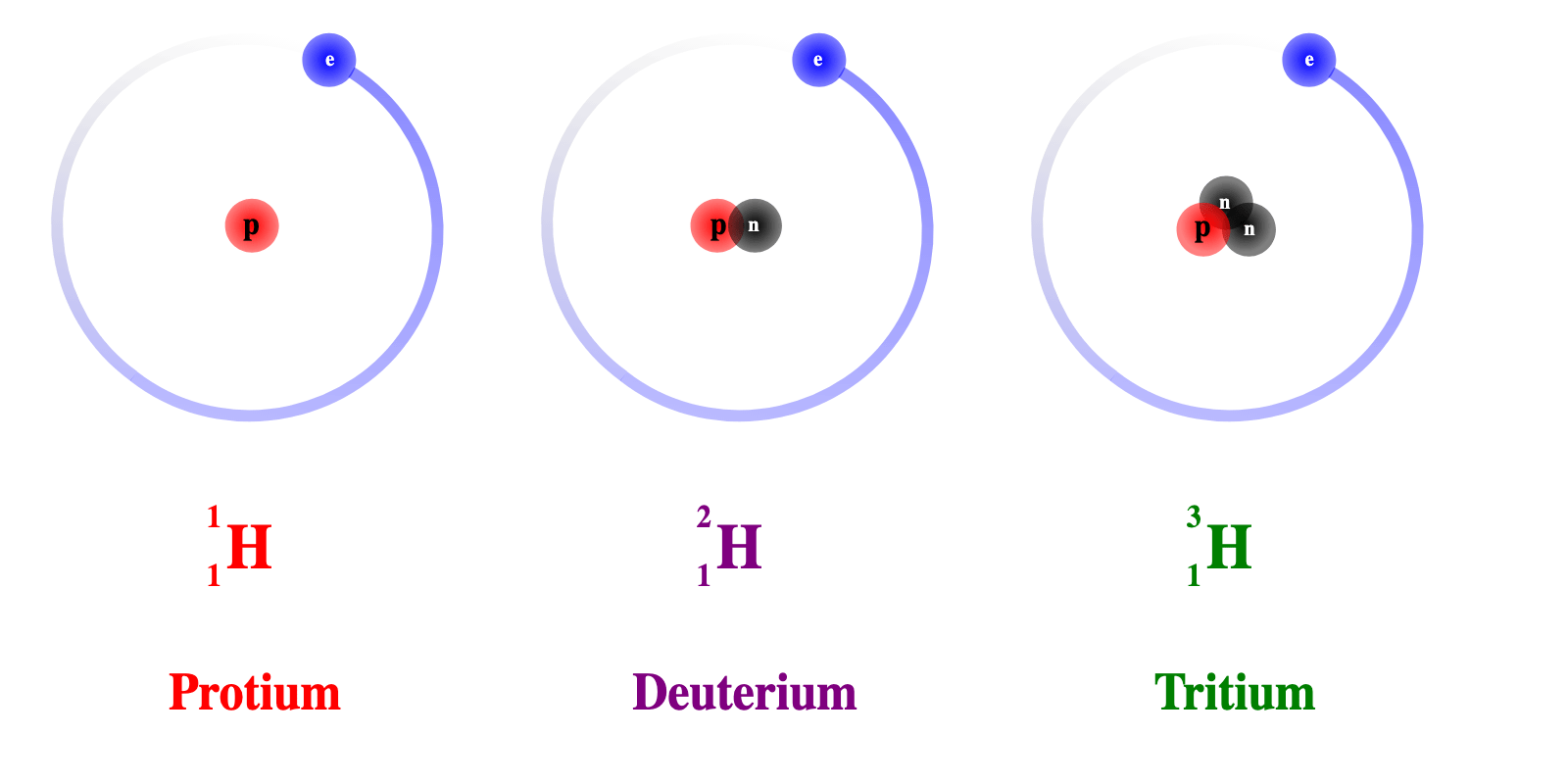
- For example, Hydrogen comes in three forms:
- Protium (normal hydrogen) with one proton and No Neutrons
- Deuterium, which has one proton and one neutron
- Tritium (artificially made), which has one proton and two neutrons
- All of these are different isotopes of the same element (Hydrogen), as shown in the image below
- The symbols of isotopes are written in the form \( \ce{^{M}_{A}E} \) where:
- M is the mass number (number of protons + number of electrons)
- A is the atomic number (number of protons)
- E is the Atomic symbol (1 or 2 letter symbol of the element)
- Isotopes can be unstable: the number of protons and neutrons in the nucleus of the atom is unbalanced, so the isotope is RADIOACTIVE
Radiation
Radiation is the emission of energy as electromagnetic waves or as moving subatomic particles, especially high-energy particles which cause ionization.
- Radiation is created when an unstable isotope emits energy in the form of Alpha, Beta or Gamma particles
- IONIZING RADIATION is any form of radiation that has the ability to make an atom ionized (positively or negatively charged)
- BACKGROUND RADIATION is any radiation that is present in the environment without adding any intentional sources
Types of Radiation
- Alpha (α) radiation (Two protons and two neutrons bound together)
- Alpha particles are created when an atom has too many protons
- The cell emits two protons and two electrons, and the resulting atom is 2 atomic numbers lower
- Alpha particles can be stopped by a sheet of paper, or by skin, or a few centimetres of air
- Beta (β) radiation (Single electron or single positron)
- Beta particles are created when an atom has too many neutrons
- One of the neutrons decays into one proton and one electron or positron
- The resulting atom is one atomic number higher
- Beta particles can be stopped by a sheet of aluminum, or 100cm of air
- Gamma (γ) radiation (high energy electromagnetic wave)
- Gamma radiation is created when an atom needs to lose excess energy
- The atom releases a high-energy photon known as a GAMMA RAY
- The atom’s atomic number and mass do not change
- Gamma Rays can be stopped by 100m thick concrete, or 40cm of lead
- Neutron (n) radiation (Single neutron)
- Neutron radiation is created as a product of nuclear fusion or fission, where the product(s) does not require the extra neutron
- The reaction releases a neutron (or multiple neutrons)
- Neutron radiation is a byproduct of nuclear reactions, so the change in atomic mass and number depends on the reaction. However, the total mass of the resulting atom(s) is one less than the total mass of the reactant(s)
- Neutron radiation can be stopped by boron, hydrocarbons, water, or large blocks of concrete
Helpful Uses For Radiation
| Uses of Radiation | Main Features (how it's used and what it's used for) |
|---|---|
| Radiotherapy | - Treats certain forms of cancer - High energy X-rays are used to kill off cancer cells |
| Smoke Alarms | - Smoke alarms use an isotope of Americium (\\(\ce{^{241}_{95}Am} \\)), which emits alpha particles - These alpha particles react with Carbon Monoxide, which is deadly to humans - When this reaction occurs, the smoke alarm's detector is triggered, and the alarm activates |
| X-Rays | - X-Rays are high energy photons, but slightly less energetic than gamma rays - This means that X-rays can pass through human bodies without harming them significantly - An X-ray machine blasts X-rays through a human body, and the X-rays that make it through are "printed" onto a phosphorus screen - Bone blocks X-rays, so wherever bone is present, the X-rays will not show up - By looking at the resulting image, any breaks or fractures will be visible |
| PET Scans | - A radioactive substance is entered into the bloodstream via IV injections - The scanner then produces a 3 Dimensional image of the blood vessels by mapping the emission of radiation that is emitted - Any internal bleeding will show up as the blood spreads out instead of following a direct path |